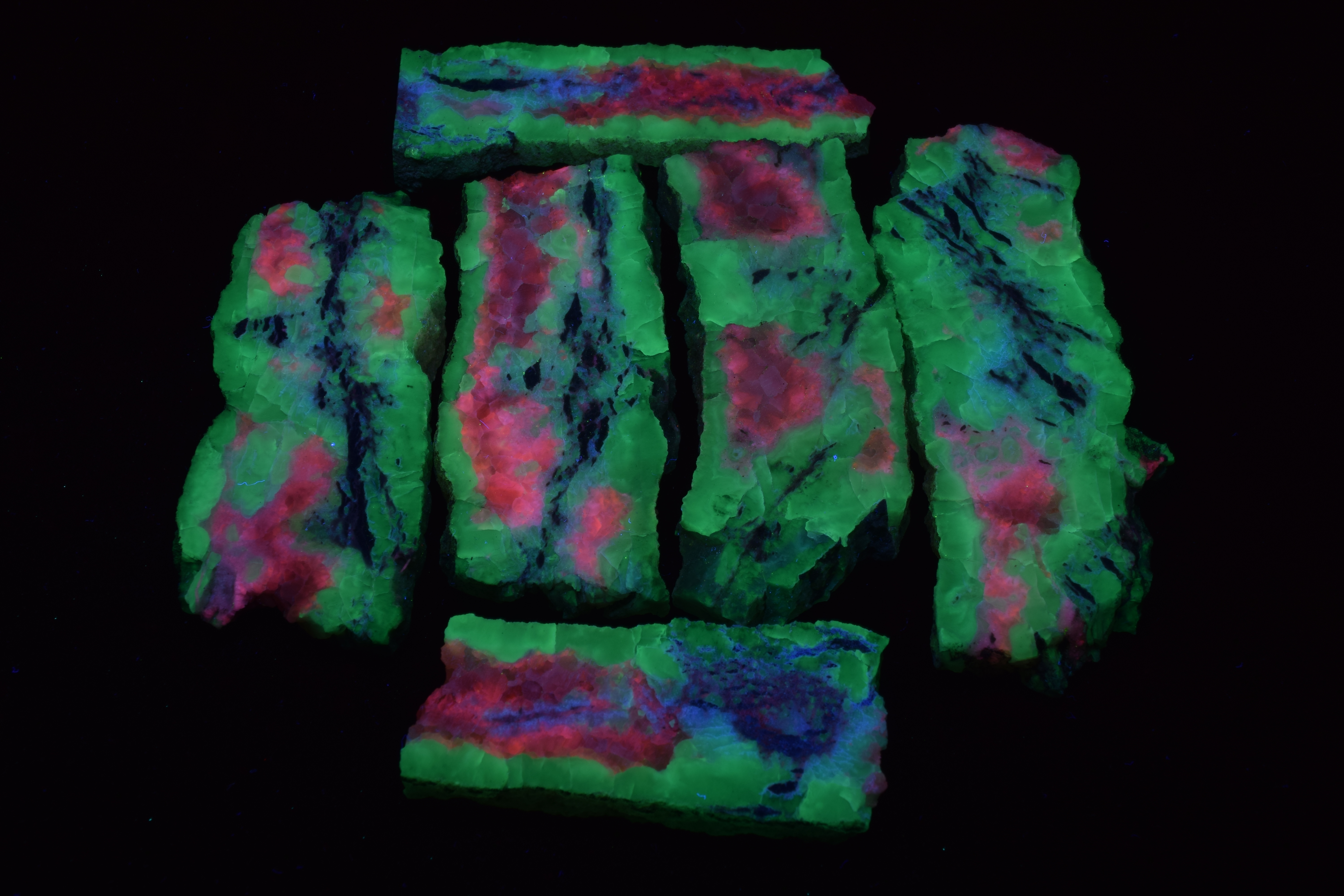
Posted by April Buettner – member of Show-Me Rockhounds of Kansas City
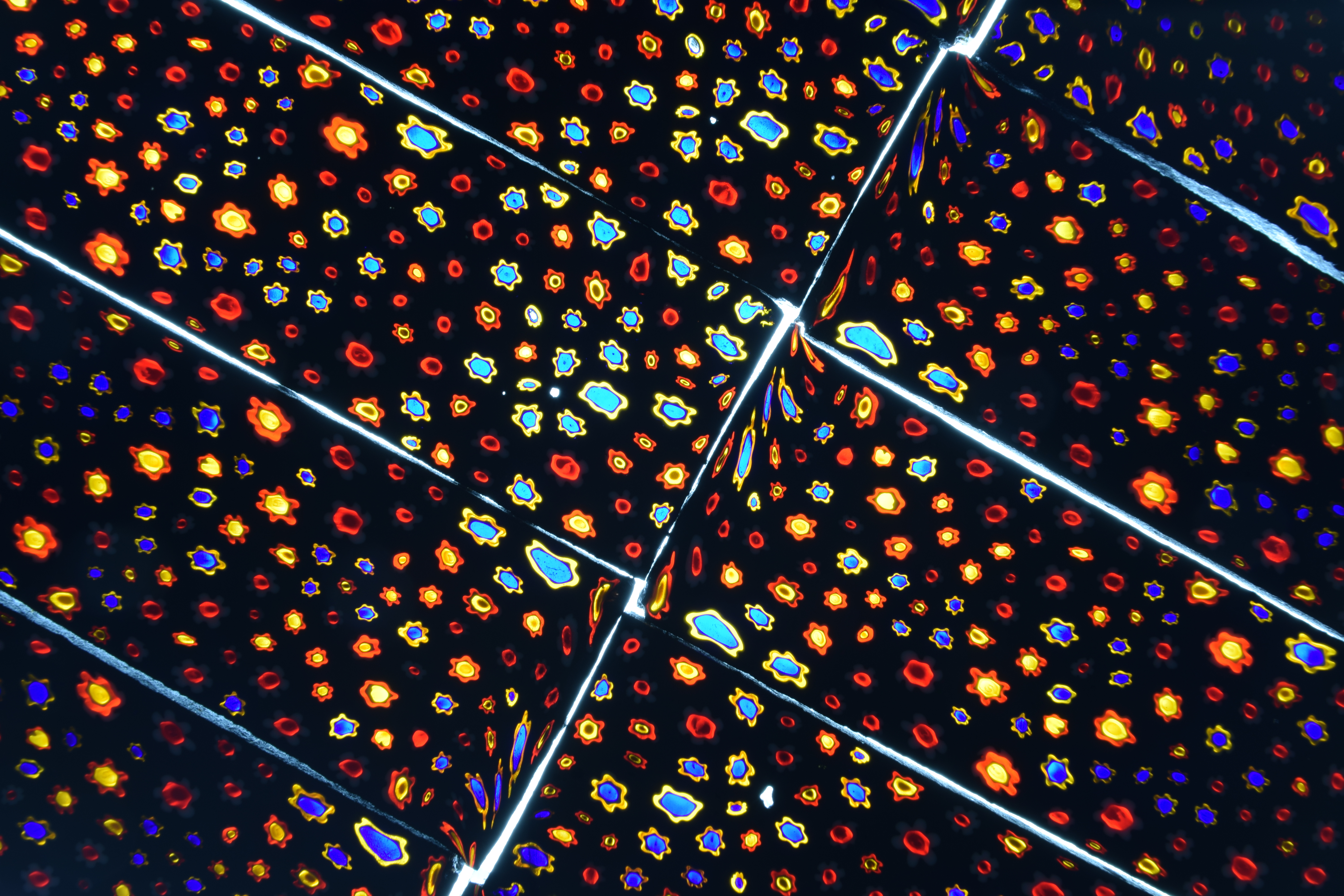
The Show-Me Rockhounds went to Baldwin City, Kansas and bought some rocks and minerals from an old time rockhound. Here are some fluorescent minerals that I bought. Photographed under short-wave UV light and white light.


From Left to Right:
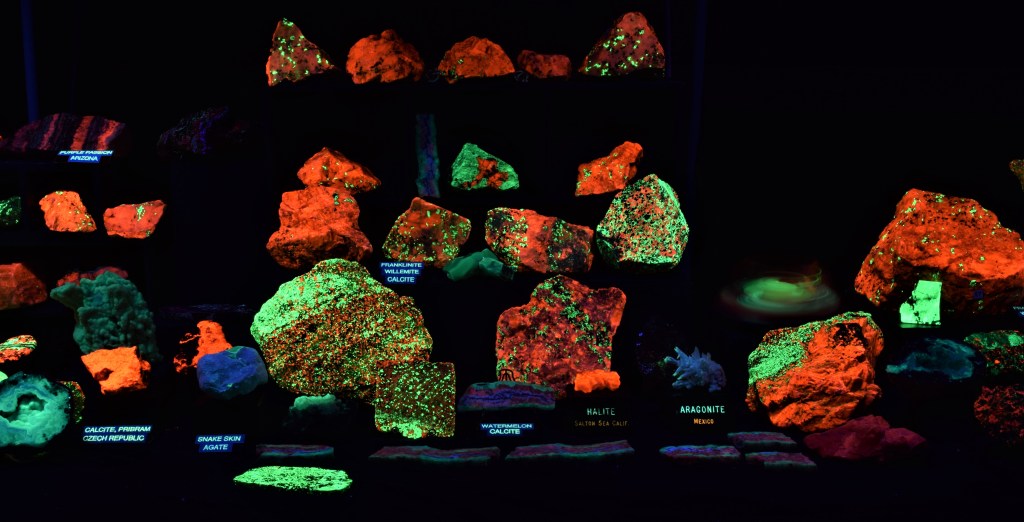
Back row: Uranyl activated Quartz; Three Purple Passion minerals from the Purple Passion Mine in Arizona. Orange is Calcite, blue is Fluorite and green is Willemite.
Middle Row: Granite with Mica; Purple Passion minerals;
Front Row: Agate; Chalcedony; Unknown “Bacon” Rock
From the collection of and photographed by April Buettner
HERE IS A VARIETY OF FLUORESCENT MINERALS THAT WILL BE AT THE 61ST GEM AND MINERAL SHOW. SPONSORED BY THE ASSOCIATION OF EARTH SCIENCE CLUBS OF KANSAS CITY. MARCH 10,11,12, 2023 AT THE KCI EXPO CENTER, 11730 AMBASSADOR DRIVE, KANSAS CITY, MO. DISPLAYED BY DAN AND CONNIE SNOW OF THE SHOW-ME ROCKHOUNDS OF KANSAS CITY.




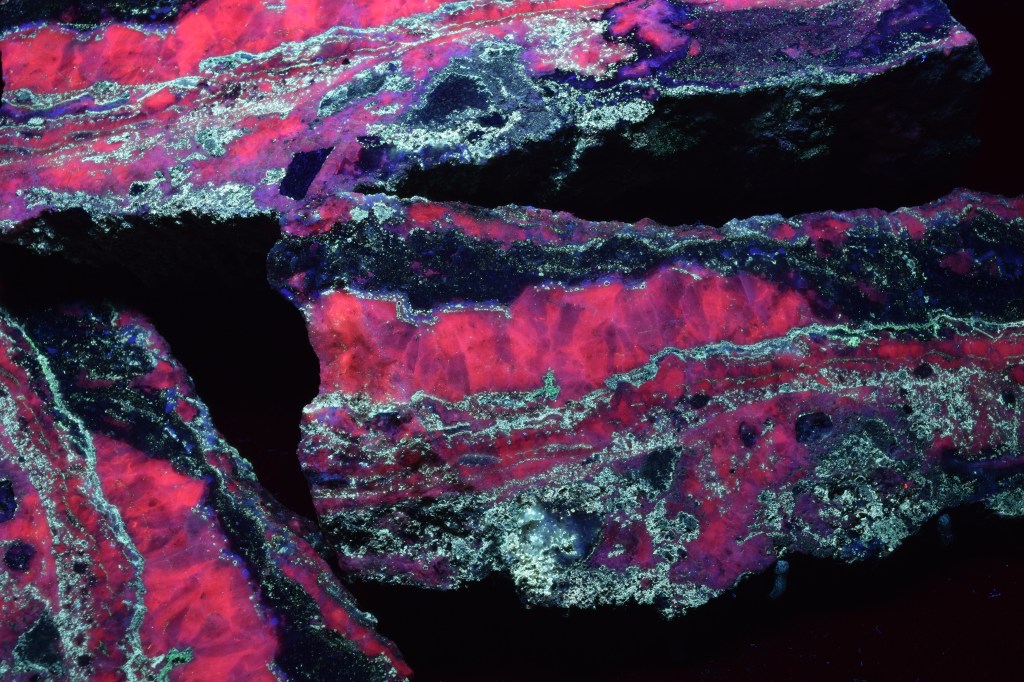


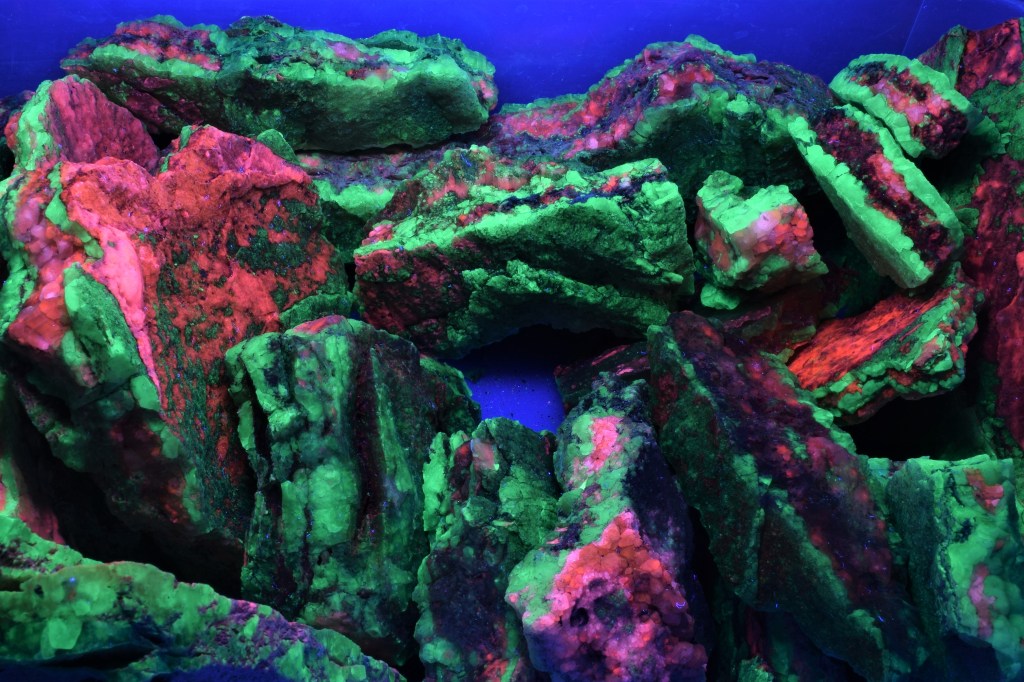
Introduction to the Mary Bergman Hamilton Collection of Minerals
In 217, the children of Mary Bergman Hamilton donated her lifetime collection of minerals to the Show-Me Rockhounds. In order to share this gift with other rockhounds and interested earth science students, our club has put together this publication.
Mary had divided her collection into minerals she had purchased, those she had traded for, and those that she had found on rockhounding field trips. We have followed this same format in how we have organized this book. At the beginning of each section we have some tips for how you, as a rockhound, can add to your collection using these methods.
A number of the minerals in the collection fluoresce under short- or long-wave ultraviolet light. We have added a section to the book to highlight these minerals and explain a little about fluorescence.
We have a page showing the collection as it was displayed at the 60th Annual Kansas City Gem and Mineral Show in 2022.
Finally, there is a page of the book to emphasize the importance of good recordkeeping and labeling of a mineral collection. We show Mary’s method of index cards and mineral labeling.
We hope that this book will inspire both novice and experienced rockhounds by showing what a dedicated amateur can accomplish.

We have books available for $15.00 per copy. If you are interested in purchasing a copy(s) you may email Dan Snow President of the Show-Me Rockhounds of Kansas City at showmerockhounds.com

THE FLUORESCENT MINERAL EXHIBIT RETURNS TO THE KANSAS CITY GEM AND MINERAL SHOW, WITH MANY ADDITIONAL SPECIMENS. SHOWINGS ARE EVERY 15-20 MINUTES, EVERY OTHER HOUR. SEE LIST OF PROGRAM HOURS. PRESENTED BY DAN AND CONNIE SNOW OF THE SHOW-ME ROCKHOUNDS OF KANSAS CITY. THIS IS SOMETHING EVERYONE WILL ENJOY.



ALL PHOTOS BY Dan or Connie Snow









PHOTOS BY DAN SNOW