




Posted by April Buettner – member of Show-Me Rockhounds of Kansas City
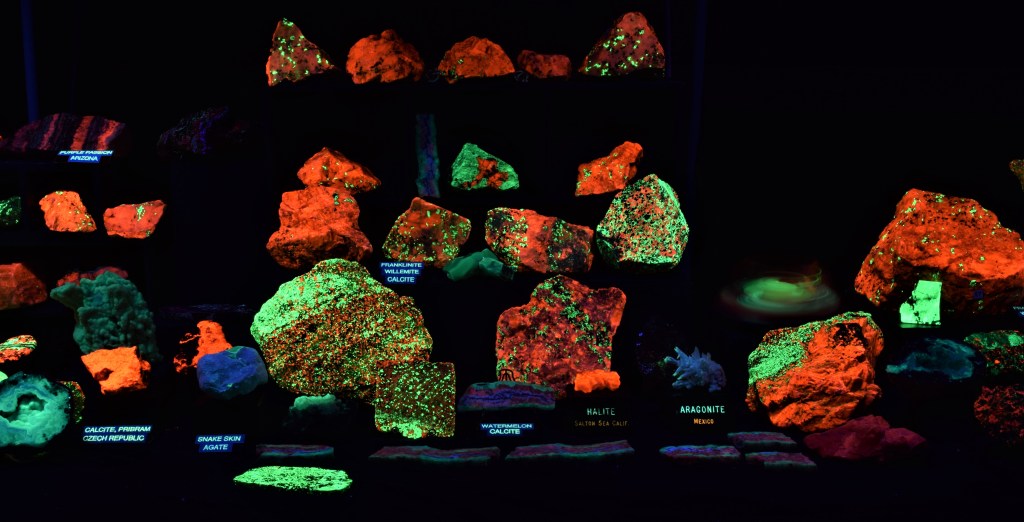
The Show-Me Rockhounds went to Baldwin City, Kansas and bought some rocks and minerals from an old time rockhound. Here are some fluorescent minerals that I bought. Photographed under short-wave UV light and white light.


From Left to Right:
Back row: Uranyl activated Quartz; Three Purple Passion minerals from the Purple Passion Mine in Arizona. Orange is Calcite, blue is Fluorite and green is Willemite.
Middle Row: Granite with Mica; Purple Passion minerals;
Front Row: Agate; Chalcedony; Unknown “Bacon” Rock
From the collection of and photographed by April Buettner
HERE IS A VARIETY OF FLUORESCENT MINERALS THAT WILL BE AT THE 61ST GEM AND MINERAL SHOW. SPONSORED BY THE ASSOCIATION OF EARTH SCIENCE CLUBS OF KANSAS CITY. MARCH 10,11,12, 2023 AT THE KCI EXPO CENTER, 11730 AMBASSADOR DRIVE, KANSAS CITY, MO. DISPLAYED BY DAN AND CONNIE SNOW OF THE SHOW-ME ROCKHOUNDS OF KANSAS CITY.




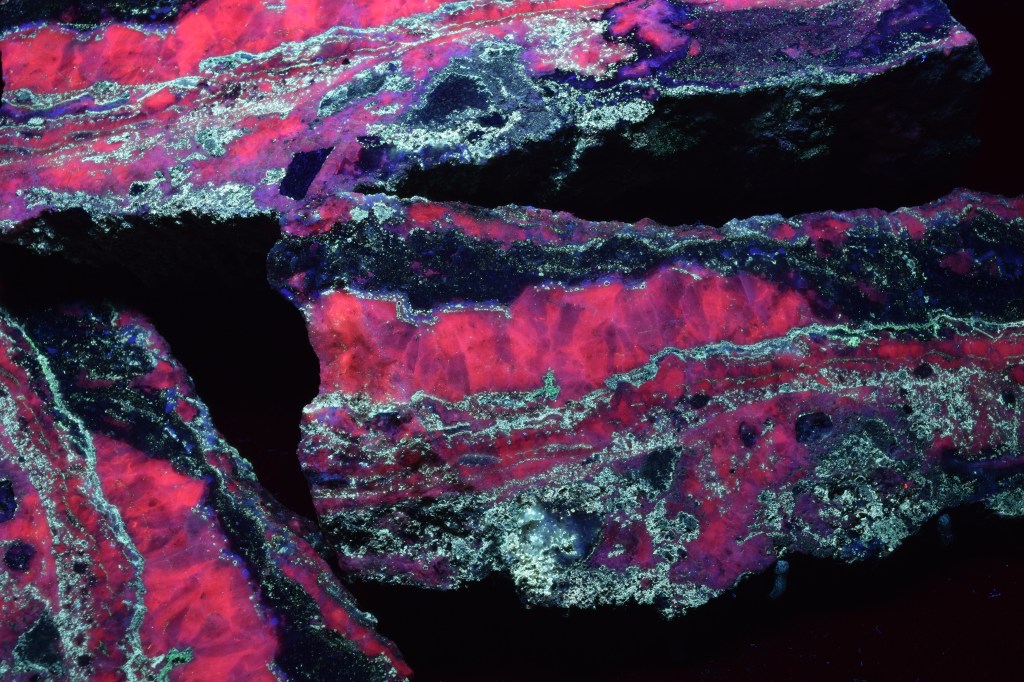


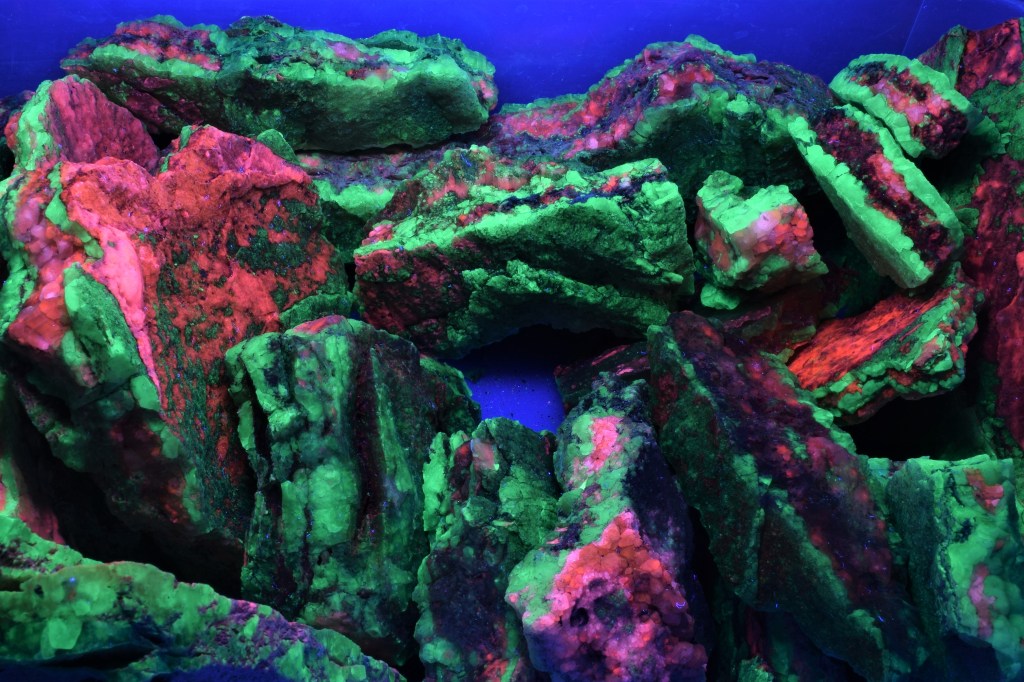

THE FLUORESCENT MINERAL EXHIBIT RETURNS TO THE KANSAS CITY GEM AND MINERAL SHOW, WITH MANY ADDITIONAL SPECIMENS. SHOWINGS ARE EVERY 15-20 MINUTES, EVERY OTHER HOUR. SEE LIST OF PROGRAM HOURS. PRESENTED BY DAN AND CONNIE SNOW OF THE SHOW-ME ROCKHOUNDS OF KANSAS CITY. THIS IS SOMETHING EVERYONE WILL ENJOY.



ALL PHOTOS BY Dan or Connie Snow








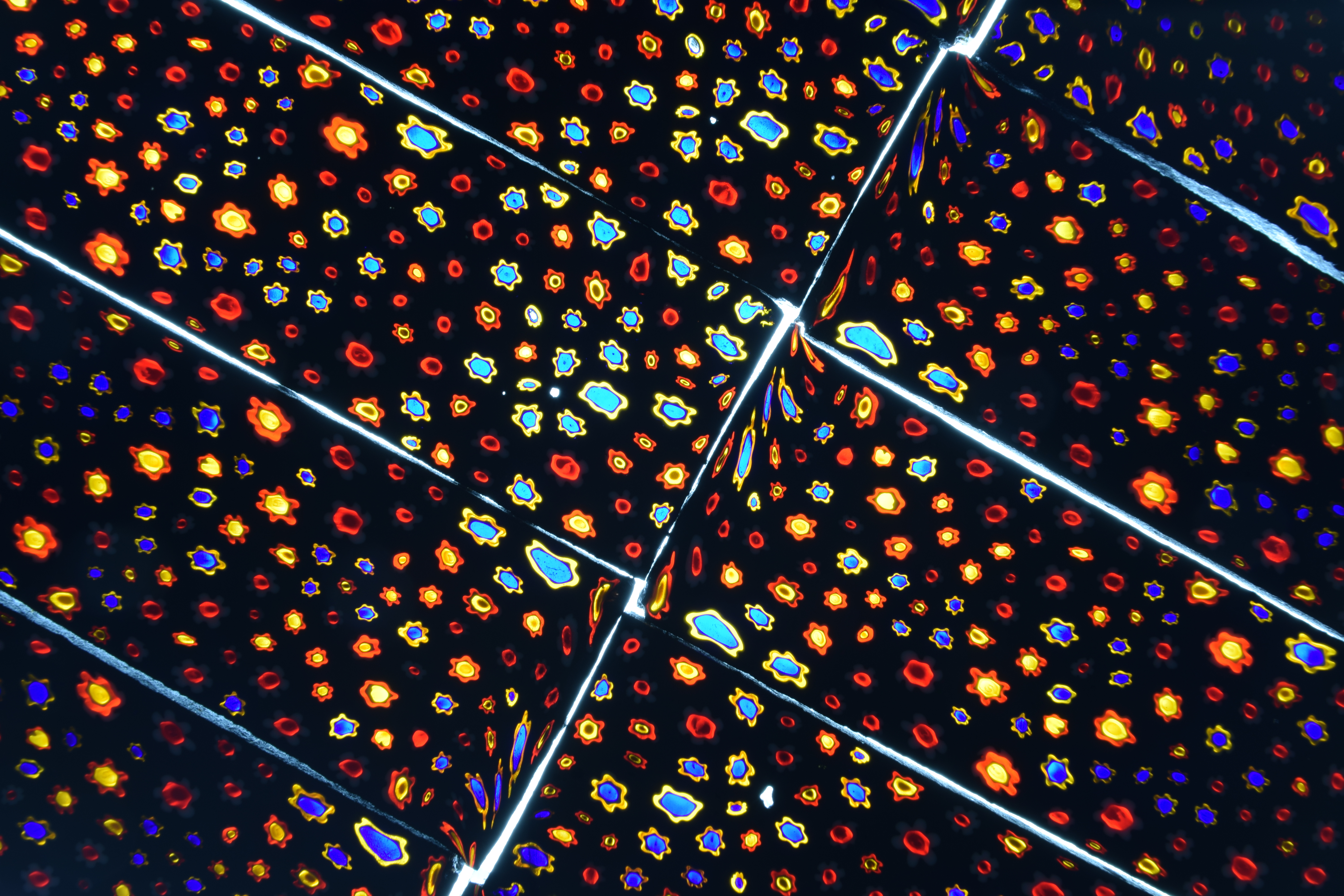

These are pictures of a few selections in our collection. The Scolecite, Stilbite-Ca and Calcite (about 12 inches long) was from Sami Makki, whose grandfather started the rudiments of Matrix India in the 50’s. Sami’s father Muhammad and he now run the operation that began exporting minerals in the 70’s. Sami personally collects much of the material and he personally collected this and other specimens we have. The specimen comes from the basalt plains in Maharashtra, India.
Sami was called by a construction crew about a potential pocket of minerals found during the digging of a well. (Sami and his team have networked with construction companies all over India and often get calls in addition to the mines they operate.) The well wall started gushing water and they quit for the day. Next day they realized they hit a pocket of water and called Matrix India to check it out before continuing to the actual water table. Sami said these Scolecite formations take millions of years of still water to form in. They then bring their own team in to remove the specimen material and reimburse the construction company.
I recently saw an article about research being done on these kinds of trapped water for bacteria that have been isolated for millions of years! The Vandinite is almost always found in oxidation zones around lead deposits (per Mindat.org). These come from Morocco and exhibit brown and reddish-brown, two of the many colors this mineral can have.


